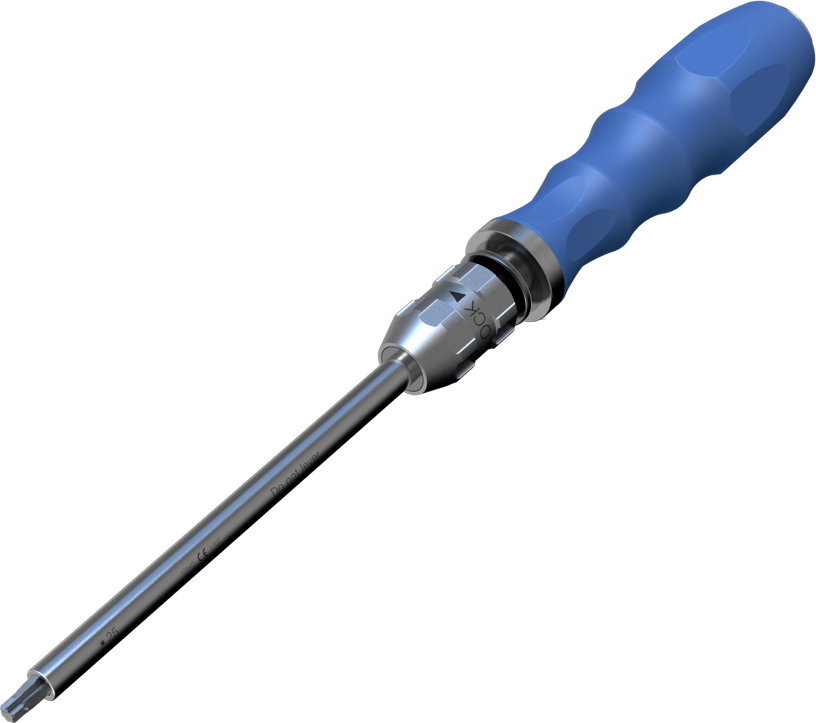
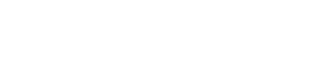Self-retaining Screwdriver


Product features

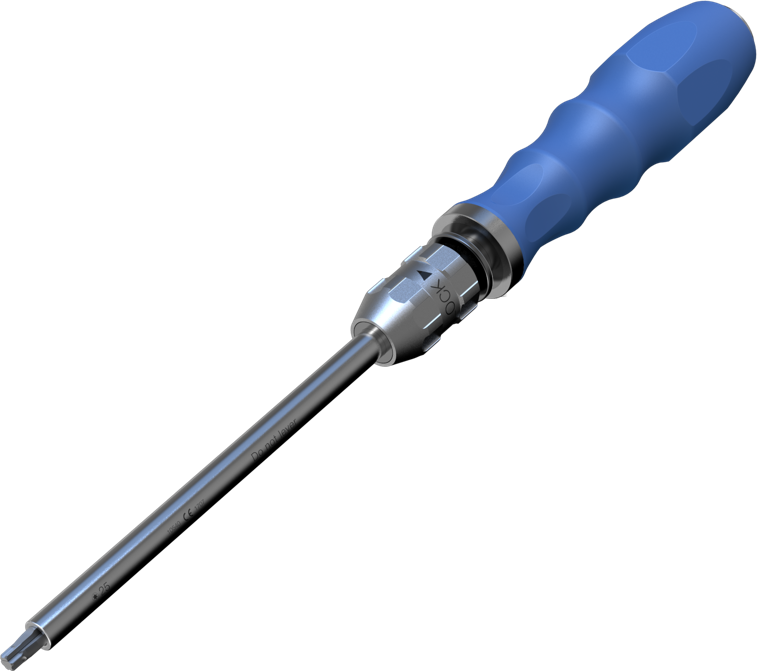
Two-part design
Adaption for handle with AO Small connection
Defined clamping and releasing of the implant
Activation time for implantation
Controlled implantation and explantation of implants
High holding power with high torque transmission


The self-retaining screwdriver has been on the global market for 13 years. So far, 32.000 units are in clinical use.
Patented self-retaining system.
In addition to a long Lifecycle, the screwdriver offers a secure locking mechanism, holds variable applications as well as ergonomic handling.
With this screwdriver, the operating time and number of instruments for the screws to be implanted or explant can be reduced.
Here you can find relevant technical documents for this screwdriver.
Self-retaining Screwdriver



Product features




Two-part design
Adaption for handle with AO Small connection
Defined clamping and releasing of the implant
Activation time for implantation
Controlled implantation and explantation of implants
High holding power with high torque transmission


The self-retaining screwdriver has been on the global market for 13 years. So far, 32.000 units are in clinical use.
Patented self-retaining system.
In addition to a long Lifecycle, the screwdriver offers a secure locking mechanism, holds variable applications as well as ergonomic handling.
With this screwdriver, the operating time and number of instruments for the screws to be implanted or explant can be reduced.
Here you can find relevant technical documents for this screwdriver.
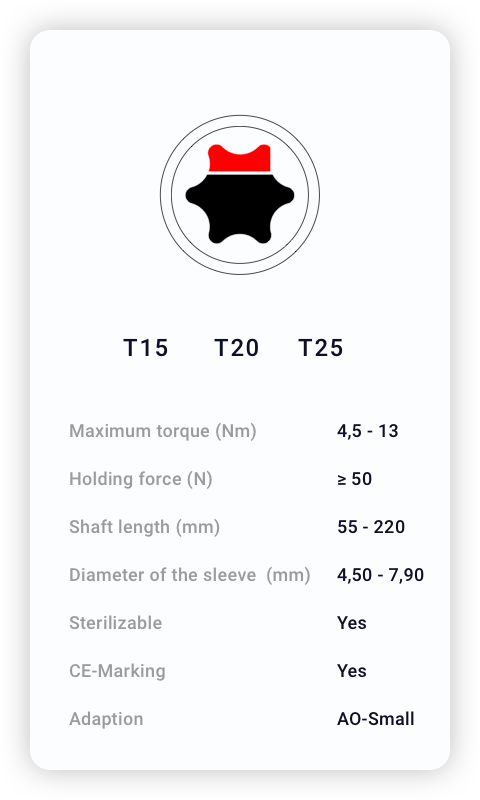
Technical Specifications
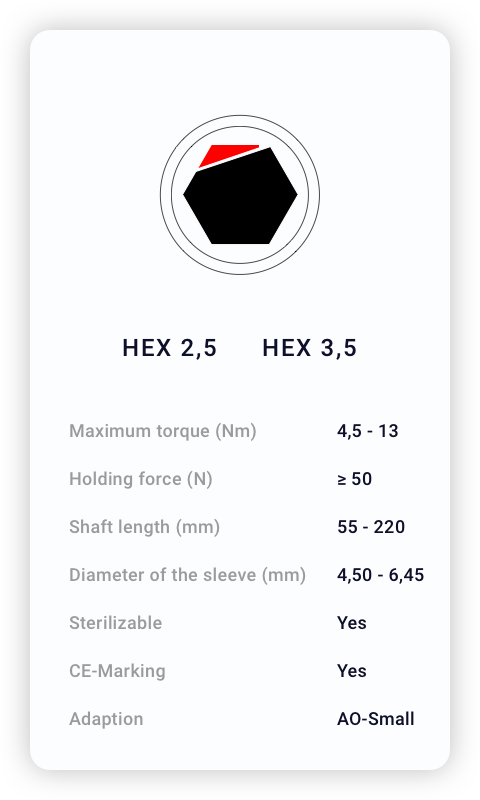
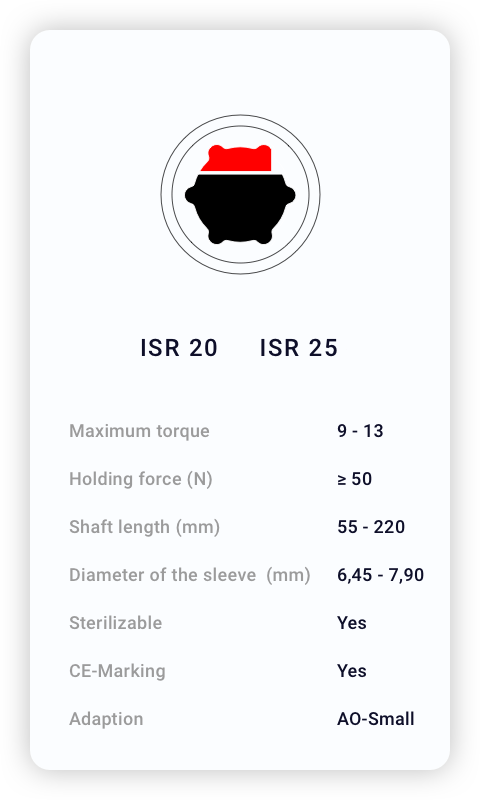
Technical Specifications



The Global Limiter
The Global Limiter (Torque Limiter) with defined torque is used in traumatology for locking implants.
The patented Global Limiter features safe handling with an ergonomic, compact and lightweight design.
Product features
- Patented torque-limiting system
- Fully integrated design
- Constant torque without readjustment
- Torque graduation in 0.1 Nm steps
- Fully sterilizable
- Integrated AO Small connection
- Clear mechanical-acoustic trigger clicks
- Handle design customer-specific
- Two designs possible
- CE-Marking

The Global Limiter
The Global Limiter (Torque Limiter) with defined torque is used in traumatology for locking implants.
The patented Global Limiter features safe handling with an ergonomic, compact and lightweight design.
Product features
- Patented torque-limiting system
- Fully integrated design
- Constant torque without readjustment
- Torque graduation in 0.1 Nm steps
- Fully sterilizable
- Integrated AO Small connection
- Clear mechanical-acoustic trigger clicks
- Handle design customer-specific
- Two designs possible
- CE-Marking



Technical Specifications


Technical Specifications


The Company
Medizin-Mechanik-Nord GmbH has been in the medical device field for 11 years developing and manufacturing high-quality products for traumatology.
The company provides many years of experience in the medical device and technology development field, and offers high-level expertise implementing technological solutions to manufacturing processes and their optimization.

Our Team


Arne Müller
Phone: (+49) 431 69126770
arne.mueller@medizin-mechanik.com
Peter Witte
Phone: (+49) 431 6902814
peter.witte@medizin-mechanik.com
The Services Offered
At Medizin-Mechanik-Nord GmbH we are committed to operating at the highest standards of corporate governance and we strive to build our partnerships based on trust and honesty.




Customer-specific requirements are structured in the development steps, under cost-conscious action, implemented on time.
Medizin-Mechanik-Nord GmbH specializes in the development, prototyping and serial production of instruments and implants.
This is realised in close cooperation with long-standing cooperation partners.
http://www.beutter.de/de/
From the idea to the finished product, all processes and optimizations are documented and archived.
In addition, each product is produced in accordance with the applicable regulations and recorded during production.
The latest process technologies and measuring- and testing systems are used.
Instruments and implants can be individually tested according to customer specifications.
Extracts are available for this purpose:
The Company
Medizin-Mechanik-Nord GmbH has been in the medical device field for 11 years developing and manufacturing high-quality products for traumatology.
The company provides many years of experience in the medical device and technology development field, and offers high-level expertise implementing technological solutions to manufacturing processes and their optimization.
Our Team


Arne Müller
Phone: (+49) 431 69126770
arne.mueller@medizin-mechanik.com
Peter Witte
Phone: (+49) 431 6902814
peter.witte@medizin-mechanik.com
The Services Offered
At Medizin-Mechanik-Nord GmbH we are committed to operating at the highest standards of corporate governance and we strive to build our partnerships based on trust and honesty.




Customer-specific requirements are structured in the development steps, under cost-conscious action, implemented on time.
Medizin-Mechanik-Nord GmbH specializes in the development, prototyping and serial production of instruments and implants.
This is realised in close cooperation with long-standing cooperation partners.
http://www.beutter.de/de/
From the idea to the finished product, all processes and optimizations are documented and archived.
In addition, each product is produced in accordance with the applicable regulations and recorded during production.
The latest process technologies and measuring- and testing systems are used.
Instruments and implants can be individually tested according to customer specifications.
Extracts are available for this purpose:




For further information or a possible cooperative partnership, please contact us.
Contact person:
Peter Witte
Russeer Weg 54a
D-24111 Kiel
Tel.: +49 431-6902814
Fax: +49 431-6902815
Mobile: +49 170-4446009
E-Mail: info@medizin-mechanik.com
Opening hours:
Monday to Friday 8:00h to 17:00h
by phone Monday to Friday 8:00h to 20:00h